

Check out how Xtampza® ER can help relieve your severe and persistent pain
A prescription medicine that can help relieve severe and persistent pain for up to 12 hours per dose.
Learn moreThe use of oxycodone medications, including Xtampza ER, may result in serious, life‑threatening, or fatal respiratory depression even when used as recommended.
The use of oxycodone medications, including Xtampza ER, may result in serious, life‑threatening, or fatal respiratory depression even when used as recommended.
What is chronic pain?
Everyone experiences pain from time to time. But chronic pain doesn’t go away. Healthcare providers define it as pain lasting longer than 3 months. Chronic pain could be caused by a health condition or injury, and sometimes there’s not an obvious cause.
But relief is possible. Your doctor chose Xtampza ER to help relieve your severe and persistent pain.
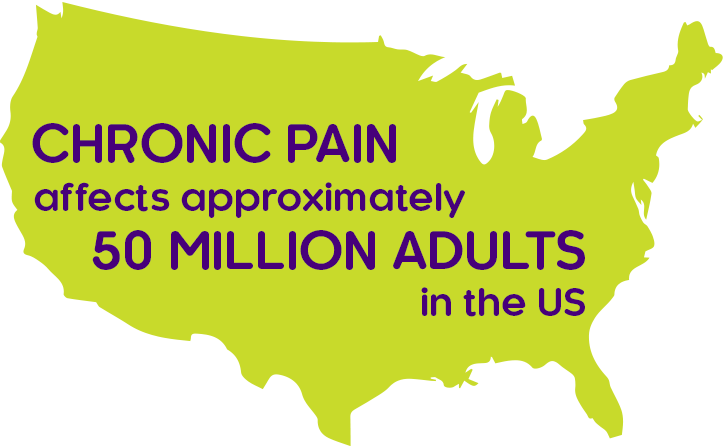
Source: Centers for Disease Control and Prevention.
What is Xtampza ER (oxycodone)?
Proven Pain Relief
Xtampza ER is an oxycodone-based pain medication. Oxycodone has been used for many years to help manage pain severe enough to require daily, around-the-clock, long-term treatment when other pain treatments, such as non-opioid or immediate-release opioid medications, do not treat your pain well enough or you cannot tolerate them. The “ER” in “Xtampza ER” stands for extended release, which means it delivers pain-relieving medication slowly over time.
Extended Pain Relief
Unlike immediate-release (IR) pain medicines that might provide short-term relief, Xtampza ER is an extended-release (ER) medication. Xtampza ER offers a dose of medication that lasts for a longer period of time in the body. ER formulations prolong pain relief over many hours, so fewer pills are needed each day. Most short-acting medications only work 4 to 6 hours at a time, which means you might have to take them up to 6 times a day. Xtampza ER provides up to 12 hours of relief with every dose, so you only take it twice a day. Xtampza ER should be taken at the same time every day and MUST be taken with food.
Flexible Administration
There are several ways you can take Xtampza ER that have been approved by the FDA. If you have difficulty or dislike swallowing pills, Xtampza ER has several options that may help.